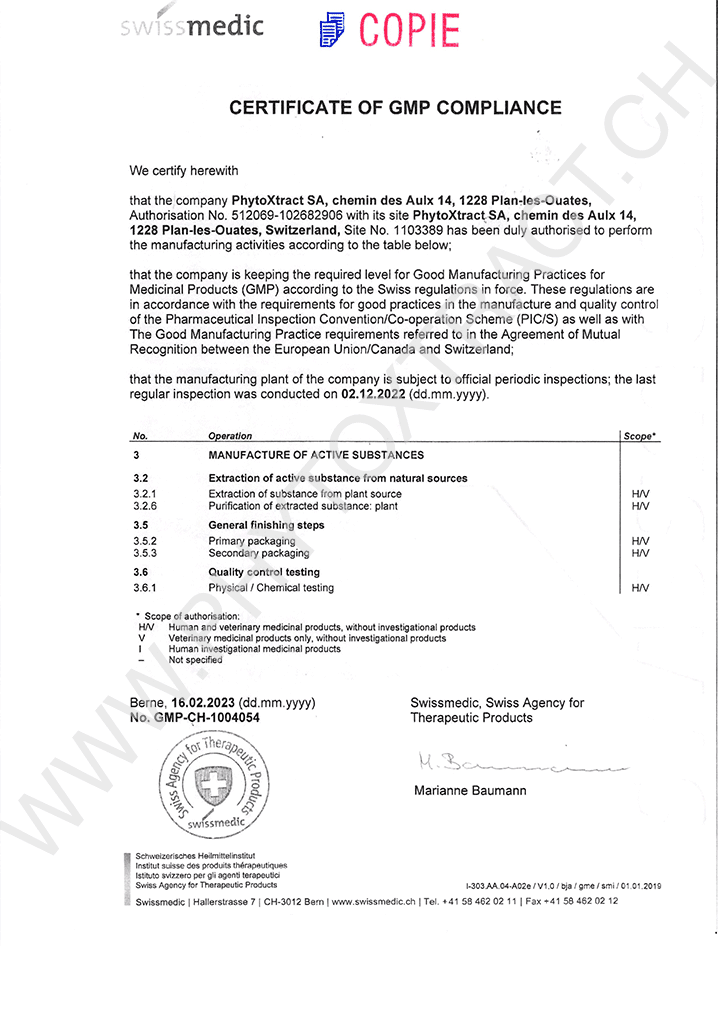
PhytoXtract SA holds an authorisation from Swissmedic, the Swiss Agency for Therapeutic Products, for the manufacture (GMP) and distribution (GDP) of medicinal products. All tasks relating to manufacture and analysis of our products are carried out in accordance with the requirements of GMP (Good Manufacturing Practices). All tasks relating to the distribution are carried out in accordance with the requirements of GDP (Good Distribution Practices).